The Martian Meteorite Compendium
Introduction to Martian Meteorites 2006
In the early 1970s, and again in the early 1990s, NASA advocated and developed plans to remotely collect samples from the surface of Mars and return them to Earth, because it is abundantly clear that much more precise and accurate observations and measurements are required than can be obtained by remote in–situ analysis (see Brett 1974, Bogard et al. 1979, Gooding 1990, Nealson et al. 1997, Neal 2000 and Agee et al. 2000). Meanwhile, 35 different meteorites have been found to have been delivered from Mars to the Earth by impacts on Mars and ejection into Earth–crossing orbits (Gladman 1997). The study of this collection of Martian meteorites provides a means to investigate Mars using modern analytical techniques originally invented to study samples returned to Earth from the lunar surface. The results of these studies also help define the requirements for missions to Mars, including the requirements for eventual sample return. Although the Martian meteorites so far identified are igneous rocks, they include some entrapped Martian atmosphere (Bogard and Johnson 1983, Leshin et al. 1996), some alteration products (Gooding 1978), some salts (Bridges et al. 2001) and possibly, some melted soil (Rao et al. 1999). There is a vast literature related to the study of Martian meteorites (see ˜2000 entries in bibliography attached). If samples are to be brought from Mars to Earth by spacecraft, it is to be expected that a similarsized effort may ensue, and clearly everything that will be learned will need to be complied, and made available electronically, in a manner similar to this Compendium.
There are now many excellent review papers dealing with many aspects of the research on Martian meteorites. Recommended reviews of Martian meteorites include those of McSween (1985, 1994), McSween and Treiman (1998), Treiman et al. (2000), Nyquist et al. (2001), Bogard et al. (2001) and Bridges et al. (2001). Swindle (2002) has recently reviewed the topic of noble gasses in Martian meteorites. Lodders (1998) calculated the average chemical composition for the data up to that point. Gibson et al. (2001) tell the "Life on Mars" story. This brief introduction does not serve as such a review.
The Mars Meteorite Compendium (MMC) is an attempt to introduce these rocks to new investigators and give
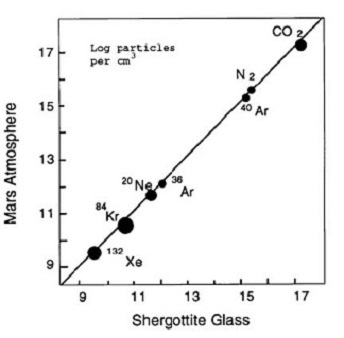
Figure 1. Log-log comparison of Vikingmeasured Mars atmosphere to trapped gases in EETA79001 glass. Figure from Pepin (1985), Nature 317, 473.
specialists a view of what is being learned by their colleagues in different fields. This compilation of data on Martian meteorites is organized rock by rock, with brief mention to each important paper according to subject (petrology, isotopes, other). The complier has found it difficult to keep up-to-date, as many new Martian meteorites have been found and many scientific studies have been reported. Thus, the MMC is now produced electronically and is continually "under construction". A complete bibliography of papers and abstracts is included. Prudent investigators should look up the original work for the original data and full discussion of ideas. Further Information outlines a handyway to view, online, many of the recent abstracts.
As early as 1872, Tschermak recognized that the Shergotty meteorite was a basalt that formed under relatively oxidizing conditions, but it wasn't until 100 years later that it became apparent to meteoriticists that the relatively
Table 1. Summary of Modal Mineralogy (volume percent)
ref. Olivine Pyroxene Plagioclase Chromite Magnetite Phosphate Sulfide Melt (maskelynite) Ti-rich (pockets) Clinopyroxenites Nakhla 1) 15 78 4 2 tr. tr. Lafayette 1) 18 70 tr. Governador 10 80 NWA 817 11) 10 69 1 Y000593 2) 10 85 MIL03346 23) 4 74 mesostasis NWA998 26) 10 70 19 other Basaltic Shergottites Shergotty 15) 0.3 71 23 2.5 1 0.3 tr. Zagami 14) 80 10 2.6 1.3 0.6 0.9 EETA79001B 18) 59 29 3.5 0.4 QUE94201 19) 43 42 1 3 6 0.24 Los Angeles 7) 41 54 1 1 NWA 480 10) 72 25 1 1 NWA 856 12) 68 23 1 2 Dhofar 378 3) 49 47 1 Y980459 24) 15.7 52.6 0.5 mesostasis NWA1669 NWA2975 25) 57.3 39.3 1.7 yes NWA3171 Lherzolitic Shergottites ALH77005 17) 60 13 9.5 2.1 0.3 13.7 LEW88516 21) 57 22 16 3 0.9 0.3 7.7 Y793605 20) 35 60 5 20 GRV99027 4) 39 55 4.4 1.1 GRV020090 NWA1950 27) 55 35 8 tr. tr. NWA2646 Olivine-phyric Shergottites EETA79001A 18) 9 70 17 3 0.2 1 DaG476 6) 15 60 15 2 1 5 SaU005 8) 25 50 15 1 0.1 0.1 5 Dhofar 019 9) 10 62 26 1.8 NWA1068 13) 27 52 16 2 NWA 1195 NWA2046 NWA2626 Dunite Chassigny 16) 92 5 2 1.4 0.3 NWA2737 22) 90 4 4.6 0.2 Orthopyroxenite ALH84001 5) 97 1 2 0.15 references: 1) Lentz 1999; 2) Mikouchi 2002; 3) Ikeda 2002; 4) Lin 2002; 5) Mittlefehldt; 6) Zipfel 2000; 7) Xirouchakis 2002; 8) Gnos 2002; 9) Taylor 2002; 10) Barrat 2002; 11) Sautter 2002; 12) Jambon 2002; 13) Mikouchi 2002; 14) McCoy 1992; 15) Stopler 1979; 16) Prinz 1974; 17) Treiman 1994; 18) McSween 1983; 19) Mikouchi 1998; 20) Kojima 1997; 21) Gleason 1997; 22) Beck 2005; 23) Mikouchi 2005; 24) Greshake 2003; 25) Wittke 2006; 26) Treiman 2005; 27) Gillet et al. 2005
young SNC meteorites may have come from the planet Mars (Walker et al. 1979, Nyquist et al. 1979). This hypothesis was explained in detail by Wood and Ashwal (1981), but it wasn't until Bogardand Johnson (1983) found gas trapped in glass inclusions in shergottite EETA79001 identical in composition to Martian atmosphere (as measured by Viking experiments), that it became widely accepted that SNC meteorites came from the planet Mars. Also in 1983, Clayton and Mayeda showed that Martian meteorites formed their own subgroup on an oxygen isotope diagram with their own fractionation line separate from that of
the Earth or HED parent bodies. In 1984, Becker and Pepin found that nitrogen isotopes and N/Ar ratios were also typical of Viking results, clinching the argument (figure 1). In 1995, Marti et al. found similar results in glass in Zagami and in 1998, Bogard and Garrison found high rare gas contents in a black glass inclusion in Shergotty as well as a second inclusion in EETA79001. Using this new data, Bogard has been able to precisely determine the isotopic composition of noble gases in the ancient Martian atmosphere.
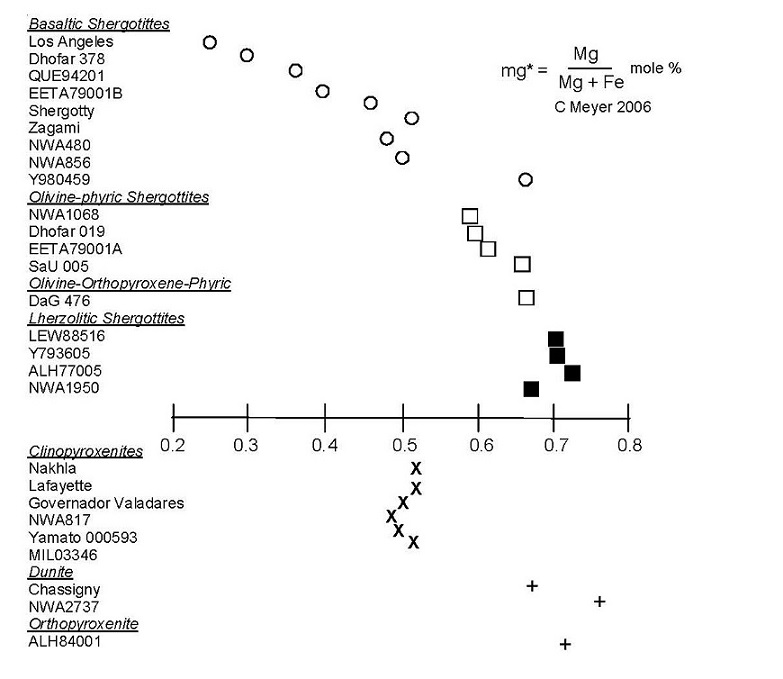
Figure 2. The Mg/Mg+Fe ratio for Martian meteorites. This index of differentiation shows that there is substantial variation in the compositions of Martian meteorites!
Briefly, Martian meteorites are igneous rocks (basalts and cumulates) that have been shocked to various degree (˜30–50 GPa). However, they are not badly brecciated, or altered. Table 1 gives a summary of the modal mineralogy. All, except one (ALH84001), have relatively young igneous ages (less than 1.3 billion years). Meteoriticists have categorized them as Nakhlites (Nakhla, Lafayette, Governador Valadares, NWA817, NWA998, Y000593, MIL03346), Basaltic Shergottites (Shergotty, Zagami, EETA79001B, QUE94201, Los Angeles, NWA480/NWA1460, NWA856, Dhofar378, Y980459, NWA1669, NWA2975, NWA3171) Lherzolitic Shergottites (ALH77005, LEW88516, Y793605, GRV99027, NWA1950, GRV99027, GRV020090, NWA2646), Olivine–phyric Shergottites (EETA79001A, Sayh al Uhamir 005, Dhofar019, NWA1068/NWA2373) and Olivineorthopyroxene–phyric Shergottites (Dar al Gani476, NWA1195, NWA2046, NWA2626) —
along with two dunites (Chassigny, NWA2737) and one orthopyroxenite (ALH84001). They range in iron and magnesium contents (figure 2). Details and variations are discussed, rock by rock, in the chapters of this compendium.
Briefly, the Nakhlites are fine–grained clinopyroxenites consisting primarily of green cumulate augite crystals with minor olivine and a minor amount of fine–grained mesostasis of maskelynite, oxides, sulfides and phosphate. Nakhlites have been found to also contain alteration products and salts from pre–terrestrial weathering. Shergottites have basaltic texture with sub-equal amounts of pyroxene and plagioclase and some mesostasis.
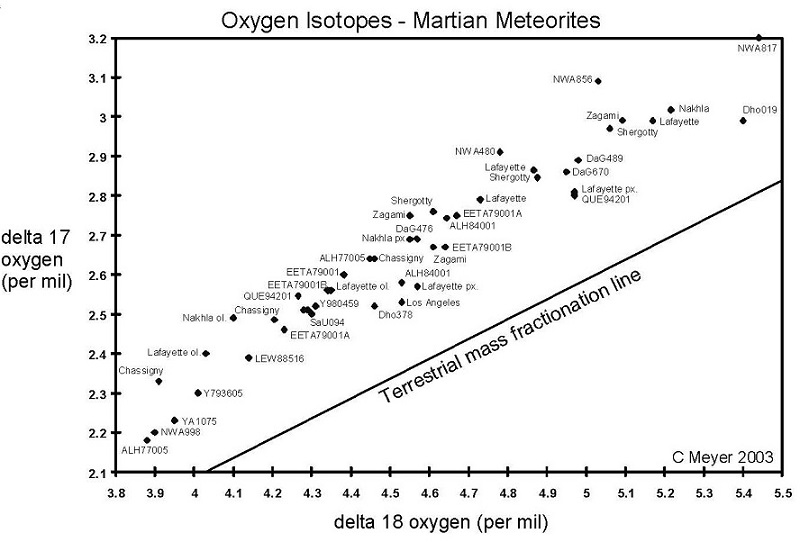
Figure 3. Oxygen isotopes of Martian meteorites. Data from various sources as tabulated under Oxygen Isotopes. Note that there is substantial variation between laboratory techniques.
Lherzolitic Shergottites contain olivine and chromite poikilitically enclosed in large orthopyoxene crystals with accessory oxides, maskelynite, sulfides and phosphates. Olivine–phyric Shergottites, contain olivine phenocrysts in a basaltic matrix (Barret et al. 2002, Goodrich 2002). Olivineorthopyroxene–phyric Shergottites contain minor, but important amounts of orthopyroxene as well (Irving 2002). The Lherzolitic and Olivine–phyric Shergottites are mafic enough to be considered peridotites. Chassigny, NWA2737 and ALH84001 are cumulates.
The Shergottites exhibit evidence of severe shock (plagioclase converted to maskelynite, brown olivine etc.). These rocks were apparently blasted off Mars by 5–8 different explosions over the last 20 million years, judging from their cosmic-ray and terrestrial exposure ages (Eugster et al. 2002). Several were seen to fall and were collected, others were recovered from the ice in Antarctica, or, more recently, desert regions in California, Africa and the Arabian Peninsula (Cassidy et al. 1992, Folco et al. 2002, Schlüter et al. 2002).
Chemical and Isotopic Signature
Martian meteorites have their own distinct chemical and isotopic signatures. The FeO/MnO ratios of rocks
and minerals are different for Martian meteorites. So are the K/La and Ga/Al ratios. But mainly the ratios of
rare gases and their isotopes are distinctive.
Perhaps the most useful are the isotopic differences. In particular, Clayton and Mayeda (1996), have shown that the Martian meteorites are distinguished by their unique oxygen isotopes, which follow a fractionation line distinct from that of the Earth and Moon or other classes of meteorites (figure 3). This unique isotopic signature for oxygen of Mars is thought to be the result of incomplete mixing in the solar nebular cloud during nebular condensation and planet formation.
The isotopic composition of weathering products and water extracted from Martian meteorites shows a contribution from the fractionated Martian atmosphere (figure 4) and the isotopic ratios of hydrogen, nitrogen and carbon also are heavy compared to the Earth,
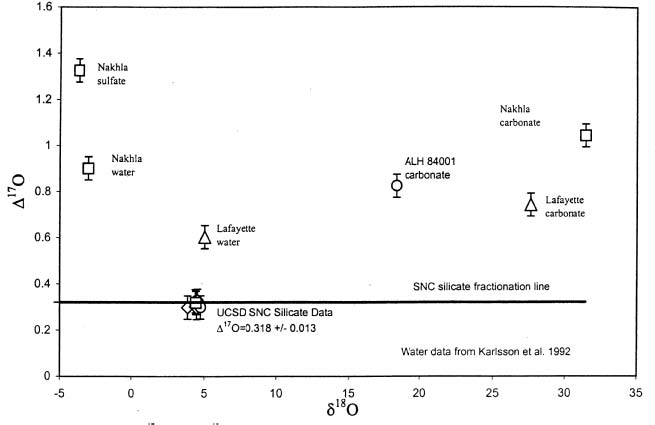
Figure 4. Oxygen isotope "disequilibrium diagram" showing that Mars has more than one isotopic reservoir (from Farquhar and Thiemens 2000, JGR 105, 11,993).
and the isotopic ratios of hydrogen, nitrogen and carbon also are heavy compared to the Earth, apparently due to atmospheric loss to space on Mars (figure 5). Even Xe isotopes (124 to 136) show mass fractionation due to atmospheric loss (figure 6). In this regard, correlated isotopic measurements, on the same sub–samples, should be a requirement for Mars Sample Return.
There is also isotopic evidence that the planet Mars formed during the interval that 53Mn (τ1/2 = 3.7 m.y.), 146Sm (τ1/2 = 103 m.y.) and 182Hf (τ1/2 = 9 m.y.) were alive (Lugmair et al. 1996, Harper et al. 1995, Lee and Halliday 1997) leaving measurable isotopic signatures (figures 7).
In 1979, Stolper pointed out the broad chemical similarity of Shergotty with terrestrial basalts (figure 8). Only a few years later, using data from Martian meteorites, Dreibus and Wänke (1985) detailed the chemical signature of Mars as distinctly different from the Earth (figure 9). They found that Mars as a planet has a relatively high abundance of volatile elements (figures 10, 11, 12). This conclusion is also reached by an analysis of the U/Th/Pb systematics (Chen and Wasserburg 1986a) which indicates a high
Pb/U ratio for source region of basalts on planet Mars. Martian rocks also have an excess of 129Xe, first seen by Rowe et al. (1966) and possibly explained by Musselwhite et al. (1991) and Marty and Marti (2002). These chemical and isotopic signatures, allow meteoriticists to group these rocks together. If one of these meteorites is from Mars, then they all are!
Mars Ejection Age
The length of time that a small rock spends in interplanetary space can be determined from its exposure to high–energy, cosmic–rays which cause
measurable changes to some isotopic ratios (e. g. He, Ne, Kr). The ejection age is the sum of the cosmic ray exposure age and the terrestrial residence time. So
far, about 5–8 groupings of ejection ages (figure 13) have been recognized (Eugster et al. 2002). Rock types and crystallization ages seem to agree with these
groupings (Nyquist et al. 2001). Two explanations of these natural groupings are possible. On the one hand, these ages could be the result of about 5–8 different
impacts on Mars within the last 20 million years.
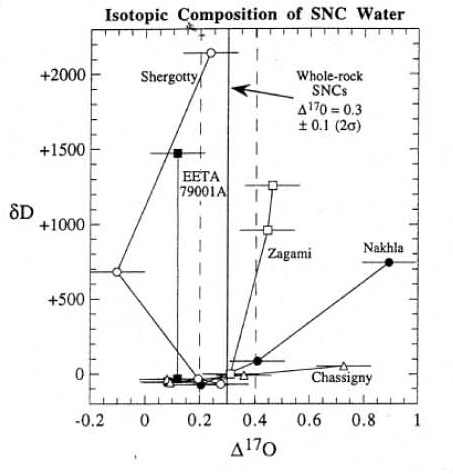
Figure 5. Hydrogen and oxygen isotopic composition of water release from Martian meteorites. This is figure 8 from Leshin et al. (1996), GCA 60, 2647.
Or, less likely, a large parent object that was ejected from Mars at earlier time could have provided shielding from cosmic rays, until these meteorites were ejected from it (Bogard et al. 1984).
Antarctic Finds
Ten of the Martian meteorites have been found on clean ice in Antarctica. In 1969, Japanese explorers found an important concentration of meteorites on blue ice
near the Yamato Mountains in Antarctica (Yoshida et al. 1971). It took until 1976, for Bill Cassidy to get support from the National Science Foundation for a
joint U.S.–Japan expedition to look for meteorites on ice within reach of the U.S. base at McMurdo. Since then, Japanese and U. S. teams with international
participation, have returned tens of thousands of meteorites from Antarctica including 9–10 from Mars, (see Yanai 1978, Lipschutz and Cassidy 1986, Cassidy
et al. 1992). Antarctica remains the best hope for finding additional pristine Martian samples.
Figure 14 shows how the ice builds up against the Trans–Antarctic Range where the katabatic winds erode the ice and leave concentrations of meteorites on the surface to be picked up by meteoriticists (figure 15). The length of time that these meteorites have been in the ice sheet is determined by the decay of several
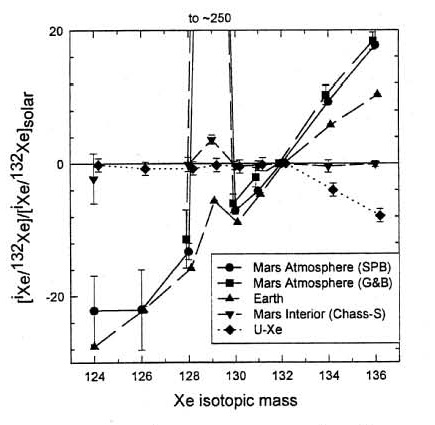
Figure 6. The Martian atmosphere has a light Xe isotopic pattern like terrestrial, but has a much larger radiogenic addition of 129Xe and is clearly distinct from the Earth in heavy Xe isotopes. This is figure 3 from Swindle (2002), Reviews in Min. and Geochem. 47, 178.
different radionuclides produced by cosmic-ray exposure in space. Radioisotopes 14C and 36Cl yield ages on the order of roughly 100,000 years.
Deserts
Following desertification, deflation caused by wind erosion can expose meteorites on the surface (e.g. Nullarbor Plain, Roosevelt County NM, Morocco,
Sahara, Oman). However, two specific conditions must be satisfied in order to successfully find meteorites in desert regions (Schlüter et al. 2002) — that is, they have
to be easy to recognize in comparison with local rocks, and they have to be preserved over a long time period.
Workshops have been held on recovery techniques for meteorites from hot and cold deserts (see Zolensky et al. 1994, Schultz et al. 1999) and we are now beginning to see results. Since 1998, numerous meteorites have been found in North Africa, including some that are from Mars and from the Moon! So far, 5 pieces of a large Olivine-phyric Shergottite have been found in southern Libya (Dar al Gani), >10 different Martian meteorites have been found in North West Africa (Morocca/Algeria ?), and 3 have been found in Oman (Sayh al Uhaymir, Dhofar). The Los Angeles shergottite was apparently found on a dry lake bed (?) in southern California.
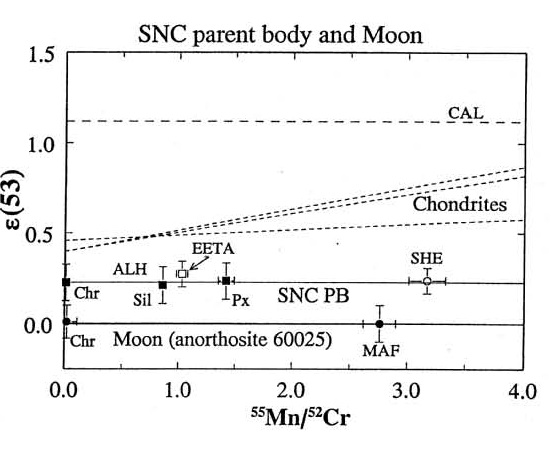
Figure 7. The isotopic composition of Cr as function of Mn content of Martian rocks is distinctly different from (Moon and Earth) (figure 10 from Lugmair and Shukolyukov 1998, GCA 62, 2863).
The desert finds are slightly weathered, but not so contaminated that they can't be used to gain scientific knowledge of Mars.
Weathering
Martian meteorites apparently have been exposed to weathering conditions on Mars as well as on Earth.
Various kinds of ‘salts’ have been found in several Martian meteorites (see Bridges et al. 2001, for review).
Gooding and his colleagues were able to show that at least some of these weathering products were present
before the fusion crust formed on the meteorites at the time of entry into Earth's atmosphere and, thus, must
have formed in an extraterrestrial (Martian) environment.
However, to complicate matters, most meteorites collected in Antarctica are weathered to some degree during their stay on Earth (Gooding 1989, Lipschutz 1982). In fact, Fudali and Schutt (1989), observed liquid water and icicles on the lee and sun facing sides of boulders at Elephant Moraine and stated that "liquid water may be a more pervasive weathering agent than previously supposed." One must be able to distinguish between terrestrial and Martian weathering.
Isotopic data are key to distinguishing which kind of weathering is dominant in a given sample. Using techniques of differential outgassing by heating,
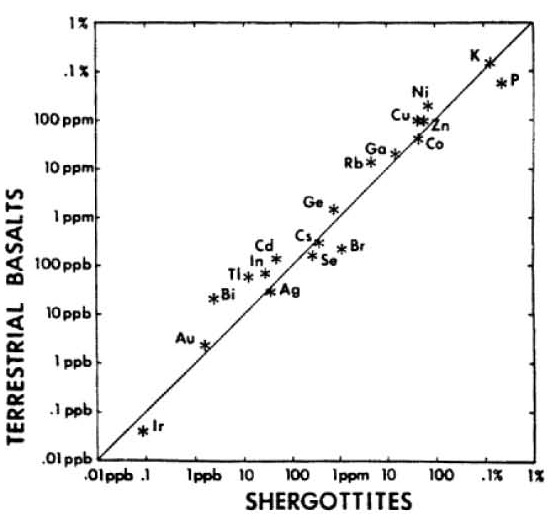
Figure 8. Comparison of composition of Shergotty with that of terrestrial basalts by Stopler (1979), EPSL 42, 239.
combustion or acid dissolution, various isotopic components have been isolated. In this regard, 14C is a useful indicator of terrestrial weathering. Jull et al. (1996) have found that 14C is correlated with other isotopic variations.
Karlsson et al. (1991) have shown that the hydrated Mg–carbonate nesquehonite forms rapidly on some meteorites found in Antarctica. The isotopic composition of carbon and oxygen (ä13C = 5.4 ‰ and ä18O = 9.4 ‰) of bicarbonate found on the chondrite LEW85320 is typical of Antarctic weathering (Jull et al. 1996). Isotopic exchange reactions also must be considered.
Martian Minerals
If one were to only consider the mineral mode (table I–1), the mineralogy of Martian meteorites may seem relatively simple. However, a wide variety of minerals
have been reported in this set of Martian rocks. Many of these minerals have only been briefly described and need to be verified. They may give a clue as to what
we are likely to find when we finally get to Mars! Some are primary (amphibole, mica, carbonate). Others are weathering products (salts). Some are shock–produced
(maskelynite, stishovite, ringwoodite). A brief tutorial of minerals found in Martian meteorites is given in Oxygen Isotopes.
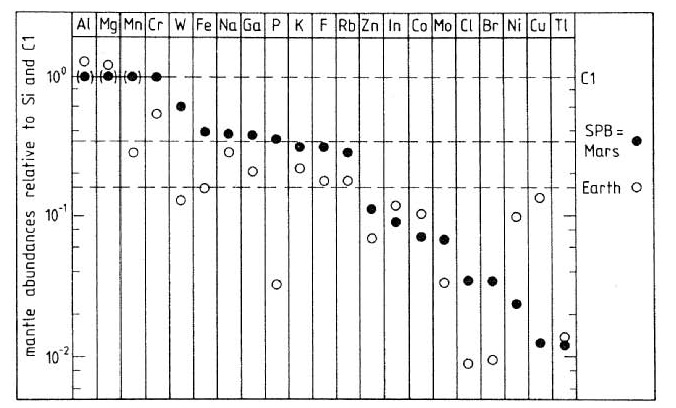
Figure 9. Comparison of major and trace element compositions of silicate portions of Earth and Mars (figure by Longhi et al. 1992, in Mars, page 194) after Wänke and Dreibus (1984). SPB = Shergottite Parent Body = SNC = Mars. C1 is chondritic composition. Mars has more volatiles (Na, Ga, K, F, Rb, Zn, Cl, Br) than Earth.
Shock
In order to eject rocks from the surface of Mars, one would think that they must be highly shocked during the impact. However, for really large impacts, Melosh
(1984, 1985) pointed out that when a rebounding shock wave traveling from the interior, reaches a free (unsupported) surface, it will accelerate and loft rocks
off the planet's surface. In this regard, it is interesting to note that Nakhla does not appear to have been highly shocked. Likewise, the delicate carbonates in
ALH84001 do not appear to have been badly damaged. On the other hand, the basaltic shergottites (Shergotty, Zagami, ALHA77005, EETA79001, LEW88516,
QUE94201 and Y793605) have all been modified by extreme shock pressures, as evidenced by shock melting of the plagioclase, fracturing, mosaicism, and
undulatory extinction of the olivine and pyroxene, polysynthetic twinning in pyroxene, oxidation in olivine and presence of melt pockets and veins (Stöffler et al.
1986, El Goresy et al. 1998, Wadhwa et al. 1994). However, shock has apparently not erased all of the original remnant magnetization (Terho 1998, Collinson
1997, Kirschvink et al. 1997).
Origins
Science is knowledge gained by the study of the behavior of nature. The intensive study of Martian
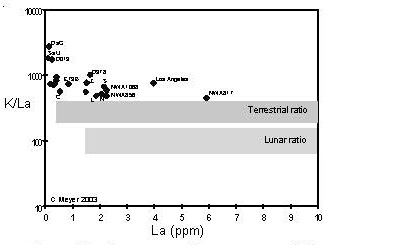
Figure 10. Comparison of compositions of K (moderately volatile) and La (refractory) largeion–lithophile elements for Martian meteorites (data from this compendium). The K/La ratios of terrestrial samples and lunar samples are given for comparison.
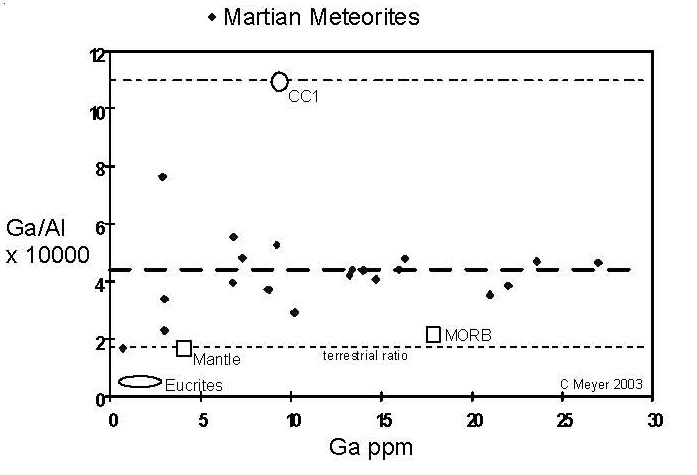
Figure 11. Comparison of compositions of Ga (volatile) and Al (refractory) elements for Martian meteorites (data from this compendium). The Ga/ Al ratios of chondrites, terrestrial and lunar samples are given for comparison.
meteorites is providing clues as to how some geological processes occur. Isotopic data (Nd, Sr) collected on Martian meteorites indicates that there is a dichotomy between the Martian crust and mantle (Borg et al. 2002). The source region of recent Martian basalts is greatly depleted in light rare–earth–elements indicating fractionation by garnet. Basalts, derived from the deep interior (mantle), become contaminated as they assimilate some of the crust as they rise from the interior and extrude on the surface (Jones 1986) and there is also evidence that they may also become slightly oxidized during assimilation of crustal material (Wadhwa 2001, Herd et al. 2002).
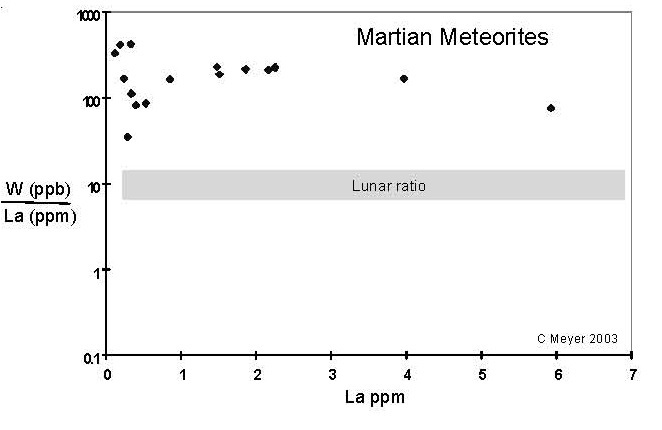
Figure 12. Comparison of compositions of W (in ppb) with La (ppm) (data selected from this compendium).
Preliminary Examination of Antarctic Meteorites Original meteorite descriptions from preliminary examination of meteorites collected by ANSMET are published in the Antarctic Meteorite Newsletter (AMN) which is a periodical issued by JSC to inform scientists of the basic characteristics of specimens recovered in Antarctica. The Smithsonian Institution (USNM) publishes meteorite descriptions and results of field investigations in their Smithsonian Contributions to the Earth Sciences. The National Institute of Polar Research (Japan) publishes catalogs (some in color) of meteorites recovered in Antarctica. Some useful cross–references to these sources are given in table 2 and under further information. During preliminary examination,
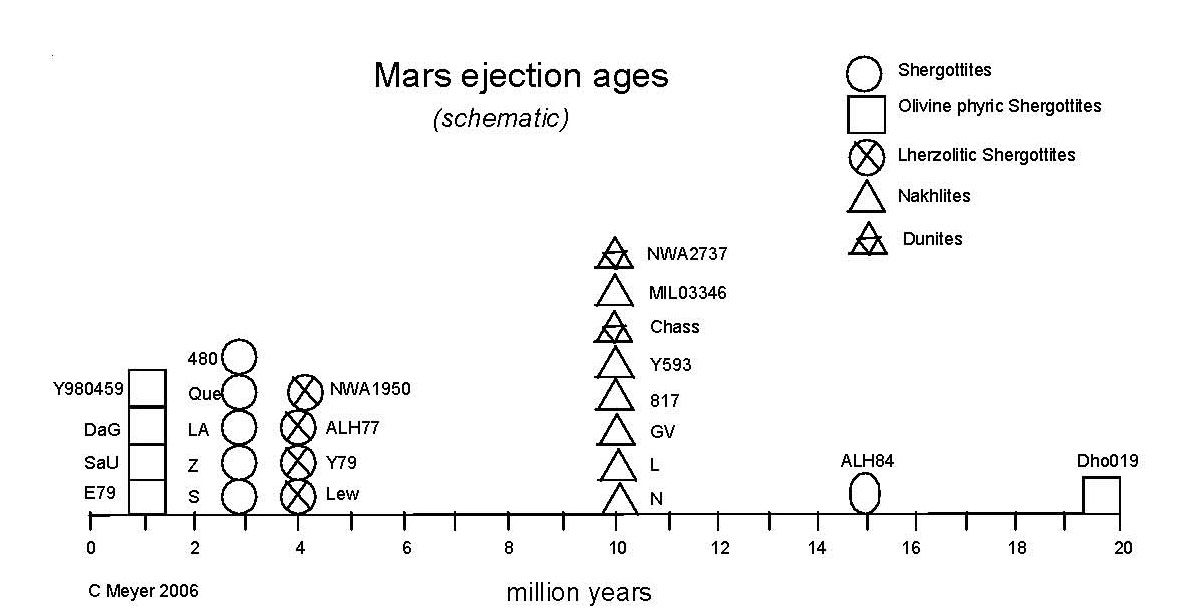
Figure 13. Histogram of "ejection ages" (averages) of Martian meteorites (from calculations by Nyquist et al. 2001, Marty et al. 2001, Eugster et al. 2002, Christen et al. 2005 and others).

Figure 14. High altitude photograph of Lewis Cliff Ice Tongue and Meteorite Moraine in Antarctica (USGS photograph TMA999–044). As the ice slides off the continent it collides with the Trans Antarctic Mountain Range, where it wells up and is ablated away by katabatic winds, leaving the meteorites concentrated on blue ice field adjacent to the mountain barriers. Approximately 20 thousand meteorites have now been collected in Antarctica, primarily by American and Japanese field parties.
Table 2. References to original rock descriptions (preliminary examination).
AMN Smith Inst. ALHA77005 1(2),9 2,339 3,048 1(3) 4(1),12 8(2),42 13(1),13 ALH84001 8(2),5 3,029 13(1),40 16(1),3 EETA79001 3(3),4 2,445 Antarctic meteorites are assigned indices of weathering and fracturing (table 3). A meteorite nomenclature committee publishes official names and descriptions of new meteorites (i.e. Grossman 1998, 2000, Russell et al. 2002). The British Museum (Natural History) maintains the Catalogue of Meteorites (Hey 1966, Graham et al. 1985, Grady 2001). 4(1),133 8(2),42 9(1) 13(1),51 LEW88516 14(2),19 QUE94201 18(2),20

Figure 15. High altitude helicopter with three meteorites on blue ice collected during 1978 joint American–Japanese expedition to Allan Hills. Picture taken by K. Yanai. NASA photo S78–28789. See nice paper by Yanai et al. (1978).
Table 3. Weathering and fracturing categories.
“Weathering” Categories: A: Minor rustiness; rust haloes on metal particles and rust stains along fractures are minor. B: Moderate rustiness; large rust haloes occur on metal particles and rust stains on internal fractures are extensive. C: Severe rustiness; metal particles have been mostly stained by rust throughout. e: Evaporite minerals visible to the naked eye. “Fracturing” Categories: A: Minor cracks; few or no cracks are conspicuous to the naked eye and no cracks penetrate the entire specimen. B: Moderate cracks; several cracks extend across exterior surfaces and the specimen can be readily broken along the cracks. C: Severe cracks; specimen readily crumbles along cracks that are both extensive and abundant.
Explanation of acronyms and jargon can be found in Acronyms and Terms. The collection, curation and allocation process for the ANSMET meteorites is briefly outlined in Steps in Antarctic Meteorite Process. The locations of field areas where Martian meteorites have been found in Antarctica
are given in Map of Antarctic Meteorite Locations. For more information on meteorites from Mars, see the websites at: https://ares.jsc.nasa.gov/ or http://www.jpl.nasa.gov/snc.
