Mare Basalt Volcanism
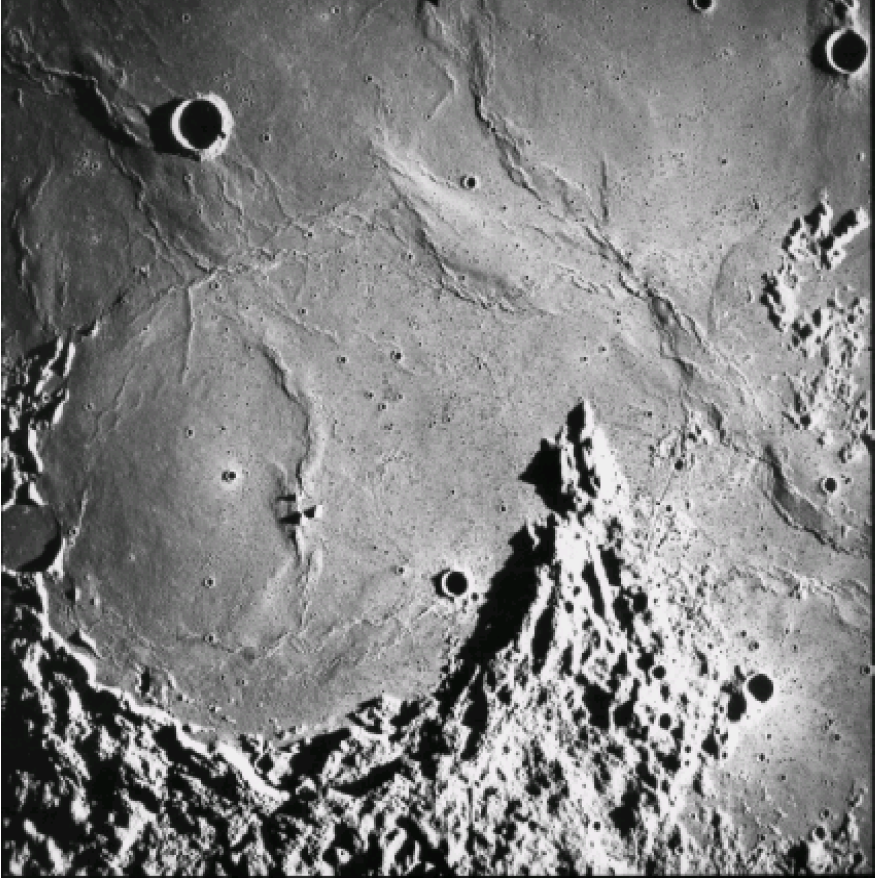
The lunar Maria are dark, low lying, and relatively uncratered. At a low Sun angle, they exhibit wrinkle ridges (fig. 9) indicating that they refilled with frozen liquid. Before we went to the Moon, we thought these Maria were relatively young because they were so poorly cratered. Surprise! The extensive collection of many, very fresh, basalt samples returned from several of the mare surfaces by the Apollo 11, 12, 15, and 17 and Luna 16 and 24 missions showed that these lunar surfaces were very old and that the cratering flux was much less than expected. In the Apollo collection, 134 samples of mare basalt are greater than 40 g; the largest sample is 9.6 kg. These basaltic samples have a wide variety of textures and compositions. Mare basalts also are represented by glass beads that have formed in basaltic lava fountains on the lunar surface. Spectral studies by Earth telescope indicate that we did not sample all the lunar basalt types (Head 1976).
Figure 9. Wrinkle ridges and flow fronts of mare basalts can be seen in Oceanus Procellarum (Letronne crater). This photo (no. 2994) was taken at low Sun angle by the metric camera onboard the Apollo 16 command and service module.
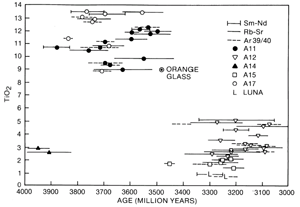
Figure 10. The ages of the Apollo 11 and 17 high-Ti basalts are older than the Apollo 12 and 15 low-Ti basalts. The age of the orange glass sample 74220 is younger than the Apollo 17 basalts and could be the cause of the dark mantle that covers the edge of Mare Serenitatis.
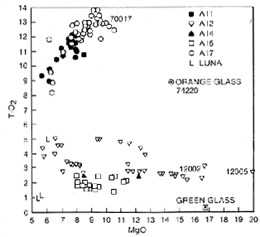
Chemically, mare basalts can be divided into two broad groups (fig. 10); the older high-Ti group (age 3550 to 3850 million years, TiO2 content 9 to 13 percent) and the younger, low-Ti group (age 3150 to 3450 million years, TiO2 content 1 to 5 percent). Samples from the Apollo 11 and 17 missions were exclusively from the high-Ti group, and samples from the Apollo 12 and 15 and Luna 16 missions were from the low-Ti group (fig. 11). The age differences and the wide variety of lunar basalt chemistries (TiO2 content from 1 to 13 percent) means that mare basalts cannot be generated from one common source region or common parental magma through different degrees of partial melting.
Figure 11. The composition of the four basalt samples in this study set compared with the composition of other lunar basalts. Compositions are given in table 4 and Basaltic Volcanism 1981.
Chemically, isotopically and mineralogically distinct source regions within the lunar interior are required. Experimental studies show that the low-Ti basalts could have been derived from an olivine-pyroxene source rock at depths ranging from 200 km to 500 km, while the high-Ti basalts could have been derived from olivine-pyroxene-ilmenite cumulates in the outer 150 km of the Moon (Papike et al. 1976).
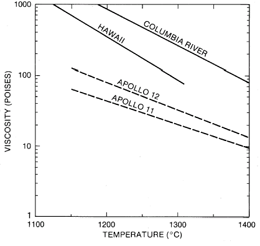
Near-surface separation of early formed olivine, ilmenite, armalcolite, metallic Fe, and/or chromite from the basaltic liquid has altered the chemistry of many rocks collected from that of the primary chemistry of the liquid that was extruded from the lunar interior. The addition or subtraction of olivine is responsible for the composition range of the low-Ti basalts, and the addition or subtraction of Fe-Ti oxides has altered the chemistry of the high-Ti basalts. Near-surface fractionation was aided by low viscosity due to the low SiO2 contents. The viscosity of mare basalt liquids (fig. 12) is much less than that of terrestrial volcanics (Weill et al. 1971).
Figure 12. Viscosity of mare basalts compared with that of terrestrial basalts. Viscosity can be calculated as a function of temperature from oxide concentrations by the methods of Weill et al. 1971.
A sequence of textural types within each basalt category represents different cooling histories. The textures range from vitrophyric to porphyritic to subophitic to intersertal to equigranular (see Definition of Lunar Terms and/or Appendix II for examples of texture). Most samples are fine-grained and have an average grain size of 0.5 mm, but some samples have phenocrysts of olivine or pyroxene measuring over 1 cm. Some samples are vesicular with interconnecting vugs and vesicles (fig. 13).
Figure 13. Vesicular mare basalt 15016. Sample is 12 cm long. See photo of thin section in Appendix 2. NASA photo no. S71-46986
Vitrophyric basalts have skeletal phenocrysts of olivine and/or pyroxene set in devitrified glass. Porphyritic basalts have partially resorbed phenocrysts set in a holocrystalline matrix. Subophitic basalts have tabular plagioclase intergrown with subhedral pyroxene. Intersertal and equigranular basalts have interconnecting anhedral crystals of plagioclase, olivine, pyroxene, and opaques. Lofgren et al. 1974 have shown that many of these textures can be reproduced experimentally. The mare basalt liquids must have crystallized rapidly if their textures can be reproduced in the laboratory!
The most important mineralogical phases in mare basalts are silicates (pyroxene, plagioclase, and olivine) and oxides (ilmenite, armalcolite, and chromite-ulvöspinel). Pyroxenes are the most abundant phase in mare basalts. Many studies of pyroxene chemical zoning were performed to try to follow the crystallization sequence and, hence, the path of differentiation of the basaltic liquids. However, pyroxene phenocrysts are complexly zoned in lunar mare basalts. Sector zoning in different crystallographic directions is beautifully illustrated in some pyroxenes. Extreme Fe enrichment, (to pyroxferroite) occurs in other pyroxenes. The plagioclase in mare basalts is very calcic because lunar samples are all very low in Na. Ilmenite, armalcolite, and chromite-ulvöspinel are abundant in high-Ti basalts. In some basalts, the olivine content is as high as 40 percent; in other basalts, there is no olivine. The mesostasis between the major minerals contains interesting accessory phases including, silica, frozen immiscible silicate liquids, troilite, metallic Fe, apatite and/or whitlockite, and a new Ti, Zr-silicate, tranquillityite. The mesostasis in lunar basalts does not alter as it would on the Earth.
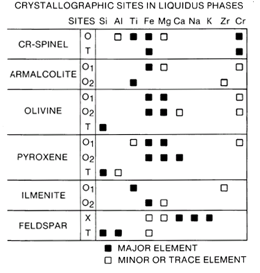
Figure 14. Distribution of elements among 14 phases in mare basalts.
Many factors influenced the mare basalt crystallization sequence. Although phase diagrams and experimental studies can be used to predict the initial crystallizing phases, metastable chemical trends in minerals and delayed nucleation of some phases often occurred as crystallization of mare basalts progressed. Figure 14 (after Papike et al. 1976) summarizes how the major elements are distributed over the various crystallographic sites in the six major minerals to crystallize from mare basalts. In this diagram, T designates the tetrahedral sites, 0 designates octahedral sites, and X designates the large cation sites in feldspar. Altogether, there are 14 different crystallographic sites among the 6 major phases crystallizing from mare liquids. Thus, the chemical composition of the crystallizing liquid (as recorded by the pyroxenes) changes in a complex manner as a result of the sequence of crystallization of phases and distribution of elements among crystallographic sites. In addition, rate processes such as diffusion in the liquid and minerals and the often incomplete reaction of early formed minerals with the liquid also apparently affected the chemical path of the crystallizing liquid. Examples of different crystallization sequences are: delayed nucleation of plagioclase in some mare basalt liquids causing a significant effect on the Ca content of the liquids and the resulting pyroxenes; partial and incomplete reaction of early formed olivine and armalcolite influencing the chemical paths of other basalts yielding free silica in their mesostasis although they were undersaturated in SiO2 initially; and extreme Fe enrichment of the residual liquid of low-Ti mare basalts which yielded pyroxferroite in the mesostasis. However, the high-Ti basalts did not yield pyroxferroite because the Fe was used up by the early-formed ilmenite.
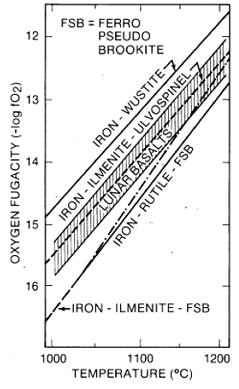
Figure 15. Partial pressure of oxygen in equilibrium with lunar basalts (from Sato et al. 1973)
Mare basalts formed and crystallized at very low partial oxygen pressures. They were in equilibrium with metallic Fe, which is found as one of their accessory minerals. Figure 15 shows the oxygen fugacity of mare basalts as a function of temperature. At 1100 deg C, the oxygen fugacity in mare basalts is about 10-13 compared with about 10-58 in terrestrial lavas. Throughout the mare basalt crystallization, water was not stable and could not have been the gas that caused vesiculation of some basalts. Under these very reduced conditions, all of the Fe in the liquid is Fe2+, most of the Cr is Cr2+, and some of the Ti is Ti3+. These reduced valence states also affected both the chemistry and stability of the minerals that crystallized from mare basalts. For example, there is no magnetite present and the only sulfide is pure troilite. Minute metallic Fe grains often are adjacent to chromite-ulvöspinel, suggesting the redox reaction Fe2+ + 2Cr2++ = 2Cr3+ + Fe during precipitation of chromite from the liquid.
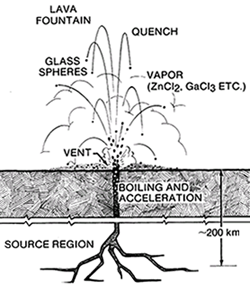
Glass droplets of possible pyroclastic origin are present in the soil samples of the Apollo 11, 15, and 17 sites (Heiken et al. 1974; Delano and Livi 1981). These are the extreme case of vesiculation (fig. 13) where the small triangles between vesicles contract by surface tension into spheres. There is no aerodynamic drag in the vacuum, so the molten droplets fall to the surface as frozen glass spheres (fig. 16). These are coated by condensed volatiles from the gas that brought them to the surface, but this gas phase is as yet unidentified (Meyer et al. 1975). Several interesting dark deposits are seen in orbital photos on the lunar surface that may represent concentrations of volcanic glasses.
Figure 16. Cartoon of a hypothetical lunar lava fountain producing glass spheres (Meyer et al. 1975). Quenched spheres become coated with volatile elements as they fall back to the surface.
Three mare basalt samples and one volcanic glass sample are included in this set of petrographic thin sections. The two Apollo 12 samples are low-Ti basalts. Although sample 12002 may represent a primitive liquid, sample 12005 is thought to be a near-surface olivine cumulate. The Apollo 17 sample 70017 is a vesicular, high-Ti mare basalt that is also thought to have been a primitive liquid. The Apollo 17 orange soil sample 74220 is the only colorful (non-gray) material that the astronauts saw on the lunar surface. This unique sample has been the object of much attention by scientific investigators. The orange glass must represent a primitive liquid derived deep in the lunar interior by endogenous partial melting (with little if any assimilation of crustal material during eruption!). Other examples of lunar basalt are found in the breccia and soil sections. Chemical compositions of the samples included in this set are given in tables 4 and 6 so that you may calculate the norm and compare with the mode.
Table 4. - Composition of Mare Basalts
| 12002 | 12005 | 70017 | 74220 | |
|---|---|---|---|---|
| SiO2 | 43.56 | 41.56 | 38.54 | 38.57 |
| TiO2 | 2.6 | 2.72 | 12.99 | 8.81 |
| Al2O3 | 7.87 | 5.3 | 8.65 | 6.32 |
| Cr2O3 | 0.96 | 0.75 | 0.5 | 0.75 |
| FeO | 21.66 | 22.27 | 18.25 | 22.04 |
| MnO | 0.28 | 0.3 | 0.25 | 0.3 |
| MgO | 14.88 | 10.07 | 9.98 | 14.44 |
| CaO | 8.26 | 6.31 | 10.28 | 7.68 |
| K2O | 0.05 | 0.04 | 0.05 | 0.09 |
| Na2O | 0.23 | 0.16 | 0.39 | 0.36 |
| P2O5 | 0.11 | 0.04 | 0.05 | |
| S | 0.06 | 0.04 | 0.16 | |
| Total | 100.43 | 99.46 | 100.59 | 99.06 |
Mineral mode
| 12002 | 12005 | 70017 | 74220 | |
|---|---|---|---|---|
| Note: Additional compositions in table 6. | ||||
| Olivine | 18 | 30 | 1 | 2 |
| Plagioclase | 18 | 11 | 26 | |
| Pyroxene | 50 | 56 | 50 | |
| Opaques | 8 | 2 | 22 | 2 |
| Mesostasis | 5 | trace | trace | |
| Silica | trace | 0 | 1 | |
| Glass | 1 | 0 | trace | 96 |