Identification of Lunar Material
The first kind of information that can be used to identify a meteorite as lunar is that obtained from either hand samples or thin sections.
Lunar meteorites can be feldspathic rocks (and including breccias), basalts (and including breccias), and mixed breccias, and each has its own specific textural characteristics. In addition, lunar materials contain some unique minerals that can help to identify them as lunar.
For example, armalcolite is a mineral first found on the Moon (Mg0.5Fe0.5Ti2O5, with a structure similar to ferropseudobrookite). In addition, some lunar basalts contain > 5 wt% ilmenite, and can also contain FeNi metal.
The second kind of information is compositional data.
The Moon is known to be depleted in volatile elements such as Na and Mn. As a result, plagioclase feldspar is highly calcic (anorthitic), and Fe/Mn ratios are higher than many other meteorites and planetary basalts.
For example, Fe/Mn ratios for lunar materials are distinct from martian and HED achondrites. This was first observed by Laul et al. (1972) and has been confirmed by many others in subsequent studies of both Apollo and Luna samples, as well as lunar meteorites (Fig. 2). Furthermore, K/La is variable in achondrites and differentiated planets (Fig. 3).
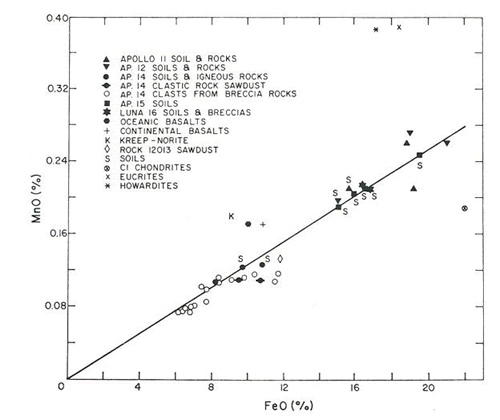
Figure 2. FeO vs. MnO correlation in Apollo samples from Laul et al. (1972); compared to eucrites, howardites, and chondrites.
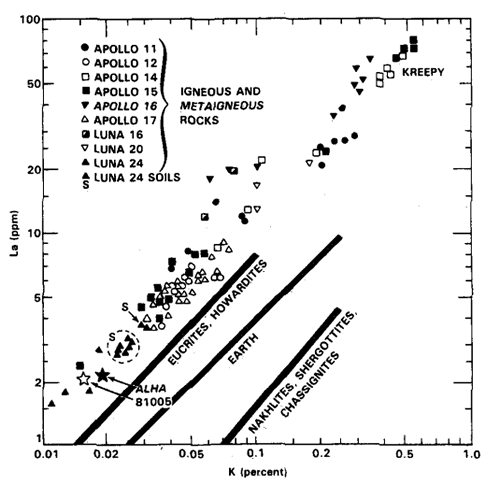
Figure 2. K vs. La correlation in Apollo samples from Wanke et al. (1972) compared to eucrites, terrestrial and martian meteorites.
The lunar K/La ratio is the lowest, and helps to distinguish lunar samples from others. This characteristic was first reported by Wanke et al. (1972). Chromium concentrations of lunar rocks are typically 100x that of equivalent terrestrial rocks (Korotev, 2005).
And finally, oxygen isotopes in material from the Moon are also distinct from other meteoritic basalts, such as eucrites and shergotttites, but identical to terrestrial samples. Mayeda et al. (1983) measured the first lunar meteorite, and a compilation of data (Table 2) shows extreme homogeneity (Fig. 4).
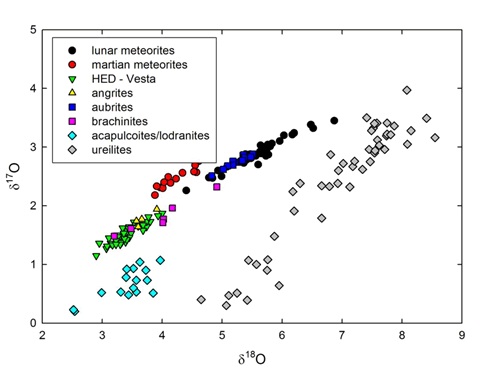
Figure 4: Three oxygen isotope diagram for lunar meteorites (data summarized in Table 2), and other achondrites (data from Clayton and Mayeda, 1996).
Table 2: Oxygen Isotopic Data for Lunar Meteorites
| Sample | Type | δ18O | δ17O | Δ17O | ref |
|---|---|---|---|---|---|
| ALH 81005 | wr | 6.03 | 3.2 | 0.0644 | 1 |
| Asuka 881757 | pc | 5.74 | 2.96 | -0.0248 | 1 |
| Asuka 881757 | px | 5.38 | 2.76 | -0.0376 | 1 |
| DaG 262 | wr (Chicago) | 5.8 | 3.01 | -0.006 | 3 |
| DaG 262 | wr (Milton-Keynes) | 6.17 | 3.21 | 0.0016 | 3 |
| DaG-400 | wr | 6.48 | 3.38 | 0.01 | 12 |
| Dho-025 | wr | 5.47 | 2.81 | -0.0344 | 2 |
| Dho-026 | wr | 5.77 | 2.87 | -0.1304 | 2 |
| Dho-287 | wr | 6.2 | 3.24 | 7 | |
| EET 87521 | wr | 5.44 | 2.83 | 0.0012 | 1 |
| Kalahari 008 | wr | 6.52 | 3.32 | 11 | |
| Kalahari 009 | wr | 6.87 | 3.45 | 11 | |
| LAP 02205 | Wr | 5.6 | 2.7 | -0.21 | 6 |
| MAC 88105 | wr | 5.73 | 2.85 | -0.1296 | 1 |
| MIL 05035 | xtals | 5.71 | 2.97 | -0.019 | 13 |
| " | matrix | 5.47 | 2.86 | -0.008 | 13 |
| NEA 001 | Wr 1 | 4.4 | 2.26 | 10 | |
| " | Wr 2 | 4.78 | 2.48 | 10 | |
| NEA 003 | wr | 5.76 | 3.04 | 14 | |
| NWA 032 | wr | 5.63 | 2.92 | -0.0076 | 4 |
| NWA 482 | Wr 1 | 4.84 | 2.47 | 10 | |
| " | Wr 2 | 5.36 | 2.73 | 10 | |
| NWA 773 | olivine gabbro | 4.99 | 2.5 | -0.0948 | 5 |
| NWA 773 | breccia | 4.93 | 2.6 | 0.0364 | 5 |
| NWA3136 | Wr 1 | 5.83 | 3.06 | -0.03 | 9 |
| " | Wr 2 | 5.96 | 3.1 | -0.05 | 9 |
| NWA3163 | Wr 1 | 5.082 | 2.663 | 10 | |
| " | Wr 2 | 5.476 | 2.833 | 10 | |
| " | Wr 3 | 5.479 | 2.809 | 10 | |
| " | Wr 4 | 5.407 | 2.785 | 10 | |
| " | Wr 5 | 5.335 | 2.782 | 10 | |
| Yamato 791197 | wr | 5.39 | 2.88 | 0.0772 | 1 |
| Yamato 793169 | wr | 5.47 | 2.88 | 0.0356 | 1 |
| Yamato 793274 | wr | 5.68 | 3 | 0.0464 | 1 |
| Yamato 82192 | wr | 5.56 | 2.85 | -0.0412 | 1 |
| Yamato 82193 | wr | 5.4 | 2.8 | -0.008 | 1 |
| Yamato 86032 | wr | 5.64 | 3.03 | 0.0972 | 1 |
| Yamato 983885 | wr | 5.65 | 2.89 | -0.05 | 8 |
| 1) Clayton and Mayeda (1996); 2) Taylor et al. (2001); 3) Bischoff et al. (1998); 4) Fagan et al. (2002); 5) Fagan et al. (2003); 6) Satterwhite (2003); 7) Anand et al. (2003); 8) Kojima and Imae (2001); 9) Kuehner et al. (2005); 10) Irving et al. (2006); 11) Sokol and Bischoff (2005) and Sokol et al. (2008); 12) Zipfel et al. (2001); 13) Joy et al. (2008); 14) Haloda et al. (2009). | |||||
